The root-mean-square speed of the molecules of an ideal gas is v.The gas is now slowly compressed to one-half its original volume with no change in temperature.What is the root-mean-square speed of the molecules now?
A) 4v
B) 2v
C) v/ 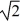
D) v
E) v/2
Correct Answer:
Verified
Q10: A fixed container holds oxygen and helium
Q11: The figure shows a graph of the
Q12: How much heat must be removed from
Q13: Oxygen molecules are 16 times more massive
Q14: Two wires are made out of the
Q16: Two containers of equal volume each hold
Q17: A 25-kg piece of equipment can be
Q18: A runner generates 1260 W of thermal
Q19: As one stretches a metal wire,which condition
Q20: A sample of an ideal gas is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents