How much heat must be added to a 8.0-kg block of ice at -8°C to change it to water at 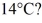
The specific heat of ice is 2050 J/kg ∙ °C,the specific heat of water is 4186 J/kg ∙ °C,the latent heat of fusion of ice is 334,000 J/kg,and 1 cal = 4.186 J.
A) 780 kcal
B) 140 kcal
C) 180 kcal
D) 810 kcal
E) 730 kcal
Correct Answer:
Verified
Q23: A person makes iced tea by adding
Q24: A metal has a latent heat of
Q25: An 920-g piece of iron at 100°C
Q26: A .20-kg ice cube at 0.0°C has
Q27: A 2,294-kg sample of water at 0°
Q29: A 35-g block of ice at -14°C
Q30: A 44.0-g block of ice at -15.0°C
Q31: A substance has a melting point of
Q32: A 45.0-kg sample of ice is at
Q33: A 400-g block of iron at 400°C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents