A substance has a melting point of 20°C and a heat of fusion of 3.4 × 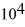
J/kg.The boiling point is 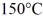
And the heat of vaporization is 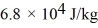
At a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid) ,1000 J/kg ∙ K (liquid) ,and 400 J/kg ∙ K (gaseous) .How much heat is required to raise the temperature of 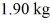
Of this substance from 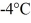
To 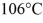
At a pressure of one atmosphere?
A) 260 kJ
B) 190 kJ
C) 230 kJ
D) 92 kJ
E) 320 kJ
Correct Answer:
Verified
Q26: A .20-kg ice cube at 0.0°C has
Q27: A 2,294-kg sample of water at 0°
Q28: How much heat must be added to
Q29: A 35-g block of ice at -14°C
Q30: A 44.0-g block of ice at -15.0°C
Q32: A 45.0-kg sample of ice is at
Q33: A 400-g block of iron at 400°C
Q34: A substance has a melting point of
Q35: If you add 700 kJ of heat
Q36: Solar houses use a variety of energy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents