For an ideal gas,
A) 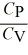 = 1 for all ideal gases.
= 1 for all ideal gases.
B) 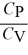 < 1 for all monatomic and diatomic gases.
< 1 for all monatomic and diatomic gases.
C) 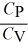 > 1 for all monatomic and diatomic gases.
> 1 for all monatomic and diatomic gases.
D) 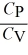 < 1 only for a monatomic gas.
< 1 only for a monatomic gas.
E) 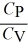 < 1 only for a diatomic gas.
< 1 only for a diatomic gas.
Correct Answer:
Verified
Q5: The second law of thermodynamics leads us
Q6: In a given reversible process,the temperature of
Q7: Which one of the following is a
Q8: Which of the following is a false
Q9: A certain ideal gas has a molar
Q11: A certain ideal gas has a molar
Q12: An ideal gas undergoes an isothermal expansion.During
Q13: An ideal gas is compressed isobarically to
Q14: For an ideal gas,
A)CP = CV for
Q15: The process shown on the TV graph
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents