The process shown on the TV graph in the figure is an 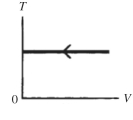
A) adiabatic compression.
B) isothermal compression.
C) isochoric compression.
D) isobaric compression.
Correct Answer:
Verified
Q10: For an ideal gas, Q11: A certain ideal gas has a molar Q12: An ideal gas undergoes an isothermal expansion.During Q13: An ideal gas is compressed isobarically to Q14: For an ideal gas, Q16: A 10-L flask and a 1-L flask Q17: When water at 0°C freezes,the entropy of Q18: An ideal gas is compressed isothermally to Q19: If the efficiency of a Carnot engine Q20: An important feature of the Carnot cycle
A) ![]()
A)CP = CV for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents