Two processes are shown on the pV diagram in the figure.One of them is an adiabat and the other one is an isotherm.Which process is the isotherm? 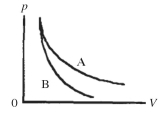
A) process A
B) process B
C) The processes shown are neither isotherms nor adiabats.
D) It is not possible to tell without knowing if the gas is monatomic or diatomic.
Correct Answer:
Verified
Q35: An expansion process on an ideal diatomic
Q36: During an isochoric process,the internal (thermal)energy of
Q37: An athlete doing push-ups performs 650 kJ
Q38: An ideal gas undergoes an adiabatic process
Q39: A fixed amount of an ideal monatomic
Q41: A refrigerator has a coefficient of performance
Q42: What is the efficiency of an ideal
Q43: An air conditioner with a coefficient of
Q44: An ideal Carnot engine operates between a
Q45: A heat engine having the maximum possible
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents