A rigid container is filled with 4.0 mol of air with CV = 2.5R.How much does the internal (thermal) energy of the air change if its temperature rises from 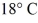
To 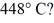
(R = 8.31 J/mol ∙ K)
A) 35,800 J
B) 430 J
C) 3,580 J
D) 8,940 J
Correct Answer:
Verified
Q61: A sealed 87- Q62: A container with rigid walls is filled Q63: A Carnot air conditioner has a coefficient Q64: How much heat is required to increase Q65: An ideal reversible heat pump is taking Q67: If we add 700 J of heat Q68: During each cycle,the compressor in a certain Q69: A heat pump with a performance coefficient Q70: An ideal Carnot air conditioner operates between Q71: The figure shows a pV diagram for![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents