For the following question(s) ,consider the following balanced equation. 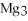
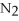 + 6
+ 6 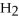 O → 3
O → 3 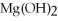 + 2
+ 2 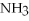
-When 4 moles of aluminum are allowed to react with an excess of chlorine gas, 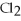 ,how many moles of aluminum chloride are produced?
,how many moles of aluminum chloride are produced?
2Al + 3 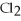 → 2Al
→ 2Al 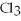
A) 1 mole
B) 2 moles
C) 3 moles
D) 4 moles
E) 5 moles
Correct Answer:
Verified
Q28: Pentane ( C5H12 )reacts with oxygen
Q37: In any balanced chemical equation,the number of
Q38: How many moles of Q41: The following reaction is an example of Q41: In an oxidation-reduction reaction,the substance oxidized always Q43: The following reaction takes place when an Q44: In a _ reaction,two or more elements Q45: In this reaction,what is the substance oxidized? Q46: For the following question(s),consider the following balanced Q47: Which of the following is an oxidation-reduction![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents