For the following question(s) ,consider the following balanced equation. 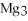
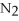 + 6
+ 6 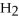 O → 3
O → 3 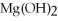 + 2
+ 2 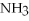
-In the reaction of nitrogen gas, 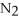 ,with hydrogen gas,
,with hydrogen gas, 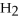 ,to form ammonia gas,NH3,how many moles of hydrogen are needed to react with two moles of nitrogen?
,to form ammonia gas,NH3,how many moles of hydrogen are needed to react with two moles of nitrogen? 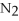 + 3
+ 3 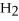 → 2
→ 2 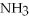
A) 2 moles
B) 4 moles
C) 6 moles
D) 8 moles
E) 5.710 moles
Correct Answer:
Verified
Q41: In an oxidation-reduction reaction,the substance oxidized always
Q41: The following reaction is an example of
Q42: For the following question(s),consider the following balanced
Q43: The following reaction takes place when an
Q45: In this reaction,what is the substance oxidized?
Q47: Which of the following is an oxidation-reduction
Q48: For the following question(s),consider the following equation.
2Mg
Q49: For the following question(s),consider the following equation.
2Mg
Q51: What is oxidized and what is reduced
Q57: In an oxidation-reduction reaction,the substance reduced always
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents