According to the Brønsted-Lowry definition,________.
A) an acid is a proton acceptor
B) a base produces 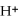 ions in aqueous solutions
ions in aqueous solutions
C) a base is a proton donor
D) a base is a proton acceptor
E) an acid acts as the solvent
Correct Answer:
Verified
Q6: The name given to an aqueous solution
Q9: Which one of the following is characteristic
Q10: Which one of the following is characteristic
Q11: According to LeChâtelier's principle,predict whether adding
Q12: The correct formula for sulfuric acid is
Q13: Which of the following is correctly identified?
A)
Q15: The conjugate acid of H2O is _.
A)
Q17: Which of the following is the strongest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents