Multiple Choice
Identify the Brønsted-Lowry acid in the following reaction.
H2O(l) + 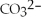 (aq) →
(aq) → 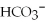 (aq) +
(aq) + 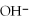 (aq)
(aq)
A) H2O
B) 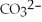
C) 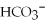
D) OH-
E) H2CO3
Correct Answer:
Verified
Related Questions
Q6: The name given to an aqueous solution
Q14: According to the Brønsted-Lowry definition,_.
A)an acid is
Q15: The conjugate acid of H2O is _.
A)
Q17: Which of the following is the strongest
Q18: For Q20: The conjugate base of H2O is _. Q21: What is the pH of a solution Q22: A solution with [ Q23: Which of the following is the correctly Q24: What is the pH of a solution![]()
A)![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents