Consider the following electronegativity values: 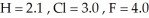 Which molecule below would you expect to have the more polar bond?
Which molecule below would you expect to have the more polar bond?
A) H2
B) Cl2
C) F2
D) HCl
E) HF
Correct Answer:
Verified
Q99: Which compound listed below will dissolve in
Q100: Which of the following has a tetrahedral
Q101: Which of the following statements are TRUE
Q102: The electronegativity value for Mg is 1.2
Q103: How many lone electron pairs does the
Q104: How does soap work?
A)Soap works by breaking
Q105: When a molecule has four electron groups
Q106: Which of the following statements about the
Q107: Which compound below is a polar molecule
Q108: The electronegativity value for N is 3.0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents