If 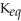 equals about ≈1,then neither direction is favored and significant amounts of both reactants and products are present at equilibrium.
equals about ≈1,then neither direction is favored and significant amounts of both reactants and products are present at equilibrium.
Correct Answer:
Verified
Q16: When dynamic equilibrium is achieved,the rates of
Q17: A reversible reaction is one that can
Q18: As long as Keq> 1,all reactants will
Q19: Dynamic equilibrium is established when the rate
Q20: Reaction rates generally increase as a reaction
Q22: If Keq = 2 for the reaction
Q23: If you have a chamber of gases
Q24: Adding heat to an exothermic reaction will
Q25: In an exothermic reaction,you can consider the
Q26: The equilibrium constant, ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents