The equilibrium constant, 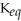 ,for an equilibrium reaction will always be the same (at a given temperature)regardless of what the initial concentrations of reactants and products were.
,for an equilibrium reaction will always be the same (at a given temperature)regardless of what the initial concentrations of reactants and products were.
Correct Answer:
Verified
Q21: If Q22: If Keq = 2 for the reaction Q23: If you have a chamber of gases Q24: Adding heat to an exothermic reaction will Q25: In an exothermic reaction,you can consider the Q27: Adding heat to an endothermic reaction causes Q28: A reaction that has Keq = 2.0 Q28: A reaction that has Keq = 2.0 Q30: Le Chatelier's principle states that when a Q31: Decreasing the volume of the system below![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents