Decreasing the volume of the system below causes the reaction to shift towards the right. 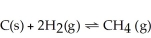
Correct Answer:
Verified
Q26: The equilibrium constant, Q27: Adding heat to an endothermic reaction causes Q28: A reaction that has Keq = 2.0 Q28: A reaction that has Keq = 2.0 Q30: Le Chatelier's principle states that when a Q32: Increasing the volume of the system below Q33: Decreasing the amount of carbon dioxide in Q34: Le Chatelier's principle states that a chemical Q35: For a given chemical equation,the coefficients for Q36: The equilibrium expression for the reaction: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents