Which of the following is TRUE of a system for which 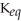 >> 1?
>> 1?
A) It will take a short time to reach equilibrium.
B) It will take a long time to reach equilibrium.
C) The equilibrium favors the reverse reaction.
D) The equilibrium favors the forward reaction.
E) none of the above
Correct Answer:
Verified
Q75: What must be TRUE for a reaction
Q76: Which of the following is TRUE of
Q77: When writing the expression for an equilibrium
Q78: For the reaction Cu2 S(s)⇌ 2
Q79: For the reaction Q81: What happens to the equilibrium position of Q82: What happens to the equilibrium position of Q83: Which compound below is the most soluble Q84: Which of the following equilibrium systems will Q85: For the reaction ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents