For the reaction 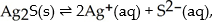 ,what happens to the equilibrium position if the amount of solid silver sulfide is doubled?
,what happens to the equilibrium position if the amount of solid silver sulfide is doubled?
A) shifts to the left
B) shifts to the right
C) does nothing
D) doubles
E) halves
Correct Answer:
Verified
Q80: Which of the following is TRUE of
Q81: What happens to the equilibrium position of
Q82: What happens to the equilibrium position of
Q83: Which compound below is the most soluble
Q84: Which of the following equilibrium systems will
Q86: Which compound below is the most soluble
Q87: What happens to the equilibrium position of
Q88: Consider the following endothermic equilibrium reaction:
Fe2O3
Q89: For the reaction Q90: For the reaction ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents