Multiple Choice
Which compound below is the most soluble in water based on its 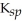 value?
value?
A) CaCO3, 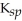 = 4.96
= 4.96 
B) MgCO3, 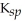 = 6.82
= 6.82 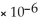
C) Fe(OH) 2, 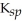 = 4.87
= 4.87 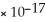
D) AgI, 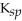 = 8.51
= 8.51 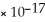
E) FeCO3, 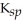 = 3.07
= 3.07 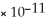
Correct Answer:
Verified
Related Questions
Q81: What happens to the equilibrium position of
Q82: What happens to the equilibrium position of
Q83: Which compound below is the most soluble
Q84: Which of the following equilibrium systems will
Q85: For the reaction Q87: What happens to the equilibrium position of Q88: Consider the following endothermic equilibrium reaction: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
Fe2O3

