At 700 K, the rate constant for the following reaction is 6.2 * 10-4 min-1. 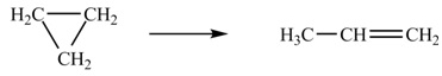 How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene
How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene
A) 1,120 min
B) 360 min
C) 3710 min
D) 1.4 * 10-4 min
E) 280 min
Correct Answer:
Verified
Q21: The half life for a first order
Q22: A reaction was experimentally determined to follow
Q23: The following initial rate data apply
Q24: A reaction was experimentally determined to follow
Q25: The isomerization of cyclopropane to form propene
Q27: A certain reaction, reaction A
Q28: A city's water supply is contaminated with
Q29: The half life for a first order
Q30: Ammonium ion (NH4+) reacts with nitrite
Q31: The isomerization of cyclopropane to propene follows
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents