For the chemical reaction system described by the diagram below, which statement is true 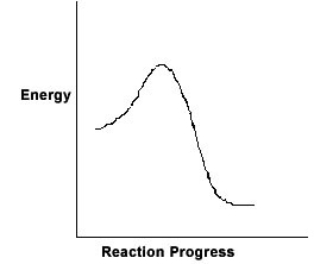
A) The forward reaction is endothermic.
B) The activation energy for the forward reaction is greater than the activation energy for the reverse reaction.
C) At equilibrium, the activation energy for the forward reaction is equal to the activation energy for the reverse reaction.
D) The activation energy for the reverse reaction is greater than the activation energy for the forward reaction.
E) The reverse reaction is exothermic.
Correct Answer:
Verified
Q62: According to the collision theory, all collisions
Q63: The activation energy for the following
Q64: The reaction C4H10
Q65: An increase in the temperature of the
Q66: For the chemical reaction system described by
Q68: Given that Ea for a certain
Q69: When the concentrations of reactant molecules are
Q70: The Arrhenius equation is k = Ae-Ea/RT.
Q71: The isomerization of methyl isocyanide, CH3NC
Q72: The following mechanism has been suggested
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents