For the chemical reaction system described by the diagram below, which statement is true 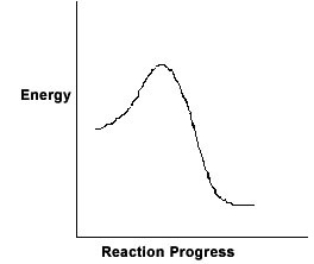 If the Ea for the forward reaction is 25 kJ/mol and the enthalpy of reaction is -95 kJ/mol, what is Ea for the reverse reaction
If the Ea for the forward reaction is 25 kJ/mol and the enthalpy of reaction is -95 kJ/mol, what is Ea for the reverse reaction
A) 120 kJ/mol
B) 70 kJ/mol
C) 95 kJ/mol
D) 25 kJ/mol
E) -70 kJ/mol
Correct Answer:
Verified
Q61: Calculate the activation energy, in kJ/mol,
Q62: According to the collision theory, all collisions
Q63: The activation energy for the following
Q64: The reaction C4H10
Q65: An increase in the temperature of the
Q67: For the chemical reaction system described by
Q68: Given that Ea for a certain
Q69: When the concentrations of reactant molecules are
Q70: The Arrhenius equation is k = Ae-Ea/RT.
Q71: The isomerization of methyl isocyanide, CH3NC
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents