What is the pressure of the gas trapped in the apparatus shown below when the atmospheric pressure is 720 mmHg ?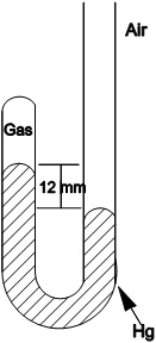
A) 12 mmHg
B) 708 mmHg
C) 720 mmHg
D) 732 mmHg
E) 760 mmHg
Correct Answer:
Verified
Q68: Which of these gas molecules has
Q69: When active metals such as magnesium
Q70: 10.0 g of gaseous ammonia and 6.50
Q71: Which gas has molecules with the
Q72: Calculate the volume of H2(g) at 273
Q74: Determine the pressure of the gas trapped
Q75: Deviations from the ideal gas law are
Q76: How many liters of oxygen gas
Q77: 10.0 g of gaseous ammonia and 6.50
Q78: For a substance that remains a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents