Determine the pressure of the gas trapped in the apparatus shown below when the atmospheric pressure is 695 mmHg. 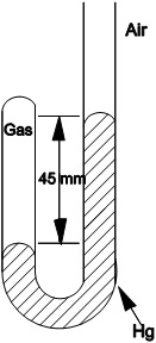
A) 45 mmHg
B) 650 mmHg
C) 695 mmHg
D) 740 mmHg
E) 760 mmHg
Correct Answer:
Verified
Q69: When active metals such as magnesium
Q70: 10.0 g of gaseous ammonia and 6.50
Q71: Which gas has molecules with the
Q72: Calculate the volume of H2(g) at 273
Q73: What is the pressure of the gas
Q75: Deviations from the ideal gas law are
Q76: How many liters of oxygen gas
Q77: 10.0 g of gaseous ammonia and 6.50
Q78: For a substance that remains a
Q79: How many liters of chlorine gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents