What is the pressure (mmHg) of the sample of gas trapped in the open-tube mercury manometer shown below if atmospheric pressure is 742 mmHg and h = 16.7 cm 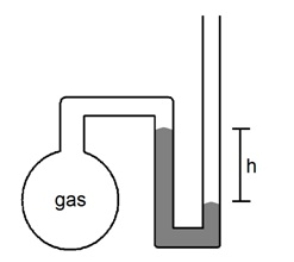
A) 375 mmHg
B) 475 mmHg
C) 575 mmHg
D) 675 mmHg
E) None of the Above
Correct Answer:
Verified
Q82: 1.000 atm of dry nitrogen, placed in
Q83: Charles Law states that the volume of
Q84: A 2.50-L flask contains a mixture
Q85: 1.000 atm of oxygen gas, placed in
Q86: What is the pressure (in atmospheres) of
Q88: What is the pressure (mmHg) of the
Q89: A "gas" is a substance in which
Q90: A spacecraft is filled with 0.500 atm
Q91: Boyle's Law states that the volume of
Q92: The magnitude of the van der Waals
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents