Which statement about the reaction below is true, given large amounts of reactants? 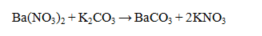
A) Ba(NO3) 2 is insoluble in water and will precipitate.
B) K2CO3 is insoluble in water and will precipitate.
C) BaCO3 is insoluble in water and will precipitate.
D) Both BaCO3 and KNO3 are insoluble in water and will precipitate.
E) All compounds in the reaction are soluble in water and no reaction occurs.
Correct Answer:
Verified
Q2: All of the following are strong electrolytes
Q3: Which compound will dissolve in water in
Q4: Which statement about bases is true?
A) Bases
Q5: Which of the following is a nonelectrolyte?
A)
Q6: Which of the following is a reducing
Q7: Which statement about the reaction below is
Q8: What is the reducing agent in the
Q9: What is the correct formula for the
Q10: What is the net ionic equation for
Q11: Which of the following best describes an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents