Hydrochloric acid solutions are often standardized by the reaction below. How many grams of CaCO3 are required to exactly react with 50.0 mL of 0.155 M HCl? 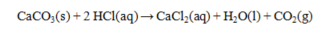
A) 0.194 g
B) 0.283 g
C) 0.341 g
D) 0.387 g
E) 0.566 g
Correct Answer:
Verified
Q49: Molarity is a unit of solution concentration
Q50: A 25.00 mL sample of H3PO4 solution
Q51: A 65.00 mL sample of HNO3 solution
Q52: How many moles of ions are in
Q53: Ammonia and sulfuric acid react according to
Q55: A solution is prepared by dissolving 20.0
Q56: How many grams of KCl are in
Q57: A 25.00 mL sample of HCl solution
Q58: A solution is made by dissolving 60.0
Q59: A neutralization reaction involves the reaction of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents