Ammonia and sulfuric acid react according to the equation given below. How many milliliters of 0.110 M sulfuric acid are required to exactly neutralize 25.0 mL of 0.0840 M NH3 solution? 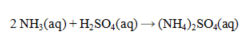
A) 1.46 mL
B) 1.82 mL
C) 3.64 mL
D) 5.85 mL
E) 9.55 mL
Correct Answer:
Verified
Q48: A 64.05 mL sample of 0.250 M
Q49: Molarity is a unit of solution concentration
Q50: A 25.00 mL sample of H3PO4 solution
Q51: A 65.00 mL sample of HNO3 solution
Q52: How many moles of ions are in
Q54: Hydrochloric acid solutions are often standardized by
Q55: A solution is prepared by dissolving 20.0
Q56: How many grams of KCl are in
Q57: A 25.00 mL sample of HCl solution
Q58: A solution is made by dissolving 60.0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents