Select the material containing the element with the highest oxidation number and determine how many electrons are required to reduce that element to an oxidation number of zero. 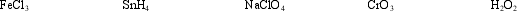
A) 8
B) 7
C) 6
D) 4
E) 3
Correct Answer:
Verified
Q3: Consider an electrochemical cell as shown, with
Q4: Which cell notation represents a battery constructed
Q5: In the salt bridge in an electrochemical
Q6: In the anode compartment of a simple
Q7: Which statement is not correct?
A) an electrochemical
Q9: Which change does not indicate a reduction?
A)
Q10: If cadmium metal and the Fe(III) ion
Q11: Which statement is correct?
A) all electrolytic cells
Q12: Which compound contains the atom with the
Q13: Which is not true of the standard
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents