Consider an electrochemical cell as shown, with Zn in ZnCl2(aq) and Cu in Cu(NO3) 2(aq) , and a salt bridge containing KNO3(aq) . The overall chemical reaction is 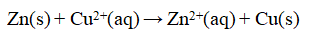
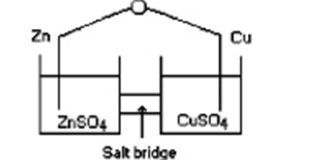
Which statement is correct?
A) one mole of electrons is transferred in this reaction
B) copper is oxidized at the anode
C) electrons travel from the Zn electrode to the Cu electrode
D) this is an example of a concentration cell
E) zinc is reduced at the cathode
Correct Answer:
Verified
Q1: The unit used to measure electrical current
Q2: The unit used to measure electromotive force
Q4: Which cell notation represents a battery constructed
Q5: In the salt bridge in an electrochemical
Q6: In the anode compartment of a simple
Q7: Which statement is not correct?
A) an electrochemical
Q8: Select the material containing the element with
Q9: Which change does not indicate a reduction?
A)
Q10: If cadmium metal and the Fe(III) ion
Q11: Which statement is correct?
A) all electrolytic cells
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents