An aqueous solution of phosphoric acid, H3PO4, contains 285 g H3PO4 in 400 mL solution, and has a density of 1.35 g/mL. Calculate
a. the weight % 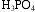 in this solution.
in this solution.
b. The concentration in mol/L of this solution.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q47: Drinkable water can be produced from seawater
Q48: The freezing points of the following aqueous
Q49: The driving force for osmosis is
A) temperature.
B)
Q50: In living systems, a solution with a
Q51: What is the vapor pressure of
Q52: A 0.1 M solution of Na2SO4 contains
A)
Q53: Consider these three primary alcohols:

Q54: The solubility in water of magnesium fluoride,
Q55: Calculate the boiling point of a
Q57: An aqueous solution at 27
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents