Multiple Choice
If the equilibrium constants for the two reactions 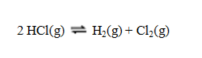 and
and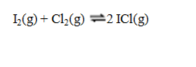
are denoted K1 and K2 respectively, then the equilibrium constant for the reaction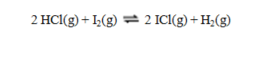 equals
equals
A) (K1/K2) 2.
B) (K1K2) 2.
C) K1K2.
D) K1 + K2.
E) K1K2/2.
Correct Answer:
Verified
Related Questions
Q10: The equilibrium constant for the reaction
Q11: In a balanced chemical equation, if there
Q12: The concentration equilibrium constant, Kc, and the
Q13: A weak acid is 5% ionized at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents