Consider the equilibrium reaction 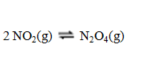 A sample of pure NO2(g) of concentration 0.140 M is allowed to come to equilibrium. It is then found that 57.0 % of the NO2(g) has reacted to form N2O4(g) . What is the value of Kc?
A sample of pure NO2(g) of concentration 0.140 M is allowed to come to equilibrium. It is then found that 57.0 % of the NO2(g) has reacted to form N2O4(g) . What is the value of Kc?
A) 0.211
B) 0.377
C) 0.754
D) 4.73
E) 11.0
Correct Answer:
Verified
Q9: The equilibrium for a particular chemical reaction
Q10: The equilibrium constant for the reaction
Q11: In a balanced chemical equation, if there
Q12: The concentration equilibrium constant, Kc, and the
Q13: A weak acid is 5% ionized at
Q15: If the equilibrium constants for the two
Q16: A chemical equilibrium may involve
A) reactants and
Q17: The equilibrium constant for a particular chemical
Q18: If a catalyst is added to a
Q19: Which of the following is not true
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents