Consider the exothermic reaction at equilibrium: 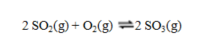 If the system is cooled, the equilibrium will ____, because ____.
If the system is cooled, the equilibrium will ____, because ____.
A) be unchanged; temperature has no effect on equilibrium
B) shift to the left; decreased temperature favors an exothermic reaction
C) shift to the right; decreased temperature favors an exothermic reaction
D) shift to the right; decreased temperature favors an endothermic reaction
E) shift to the left; decreased temperature favors an endothermic reaction
Correct Answer:
Verified
Q35: For the reaction Q36: Consider the equilibrium system Q37: A particular reaction mixture (Kp = 10) Q38: Consider the reaction Q39: Consider the reaction Q41: If the value of Kc for a Q42: This reaction is the basis for the Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()

