The reaction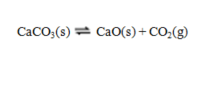 is the basis of a very ancient chemical industry. Seashells or chalk (limestone) are roasted in kilns to produce CaO (quicklime). When moistened and used in a mortar, this forms Ca(OH)2 (slaked lime). Atmospheric CO2 slowly converts this back to CaCO3. A strong bond thus results between the stones used in the wall.
is the basis of a very ancient chemical industry. Seashells or chalk (limestone) are roasted in kilns to produce CaO (quicklime). When moistened and used in a mortar, this forms Ca(OH)2 (slaked lime). Atmospheric CO2 slowly converts this back to CaCO3. A strong bond thus results between the stones used in the wall.
a. Is the above reaction exothermic or endothermic? Explain.
b. Lime kilns, since ancient times, have been designed with efficient chimneys to draw exhaust gases away. In terms of Le Chatelier's Principle, give two reasons why this enhances the conversion of limestone to quicklime.
c. When a sample of CaCO3(s) is placed in a sealed, evacuated container, the equilibrium CO2 pressure at a given temperature is always the same, and is not influenced by the amount of CaCO3, provided that there is still some present at equilibrium. Explain why.
d. How would the result in Part c be affected by adding an equal number of moles of CaO to the flask? Explain.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q38: Consider the reaction Q39: Consider the reaction Q40: Consider the exothermic reaction at equilibrium: Q41: If the value of Kc for a Q42: This reaction is the basis for the Q44: For an exothermic reaction, an increase in Q45: If a reaction is product-favored on the Q46: Consider the reaction Q47: Which statement concerning product-favored reactions is not Q48: Which of the following is true for Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

