Consider the reaction
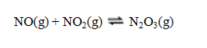 for which Kp = 2.00 at 20 C. A mixture of NO(g) at a partial pressure of 1.50 atm and NO2(g) at a partial pressure of 0.50 atm is allowed to come to equilibrium in a sealed container maintained at 20 C. What is the total pressure now?
for which Kp = 2.00 at 20 C. A mixture of NO(g) at a partial pressure of 1.50 atm and NO2(g) at a partial pressure of 0.50 atm is allowed to come to equilibrium in a sealed container maintained at 20 C. What is the total pressure now?
The expression for 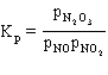 .
.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q41: If the value of Kc for a
Q42: This reaction is the basis for the
Q43: The reaction Q44: For an exothermic reaction, an increase in Q45: If a reaction is product-favored on the Q47: Which statement concerning product-favored reactions is not Q48: Which of the following is true for Q49: Consider the statement, "At equilibrium, a reaction Q50: In predicting which side of an equilibrium Q51: Consider the reaction![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents