Consider the reaction 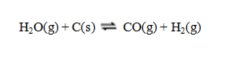 Consider an equilibrium mixture of these substances. If the pressure is increased by compressing the mixture, equilibrium will ____; if pressure is increased by adding an inert gas to the mixture, equilibrium will ____.
Consider an equilibrium mixture of these substances. If the pressure is increased by compressing the mixture, equilibrium will ____; if pressure is increased by adding an inert gas to the mixture, equilibrium will ____.
A) shift to the left; shift to the left
B) shift to the right; shift to the left
C) shift to the left; remain unchanged
D) shift to the right; remain unchanged
E) shift to the right; shift to the right
Correct Answer:
Verified
Q47: Which statement concerning product-favored reactions is not
Q48: Which of the following is true for
Q49: Consider the statement, "At equilibrium, a reaction
Q50: In predicting which side of an equilibrium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents