Concerning the Haber-Bosch for the synthesis of ammonia, 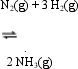 which of the following is true?
which of the following is true?
A) the reaction is strongly exothermic
B) the reaction is carried out at high pressure
C) ammonia is continually liquefied and removed from the process
D) the temperature is raised because the reaction is too slow at room temperature
E) all of these
Correct Answer:
Verified
Q47: Which statement concerning product-favored reactions is not
Q48: Which of the following is true for
Q49: Consider the statement, "At equilibrium, a reaction
Q50: In predicting which side of an equilibrium
Q51: Consider the reaction Q52: For a particular chemical reaction, which of Q53: Considering only the probability factor in the Q54: Consider the reaction Q55: As the temperature is increased, which kinds Q56: If the value of Kc for a
![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents