Select the correct Lewis structure for nitrogen trifluoride, NF3.
A) 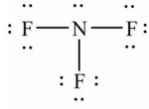
B) 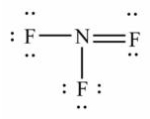
C) 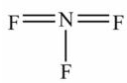
D) 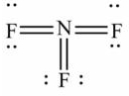
E) 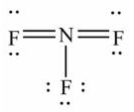
Correct Answer:
Verified
Q3: What does the abbreviation VSEPR stand for?
A)
Q4: What is the predicted molecular geometry of
Q5: How many lone pairs of electrons need
Q6: Select the correct Lewis structure for NOCl,
Q6: Which of the following is required for
Q9: According to the VSEPR model, the predicted
Q13: The Lewis structure for CS2 is:
A)
Q60: The number of resonance structures for the
Q66: How many total resonance structures can be
Q77: The total number of lone pairs in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents