For the reaction at equilibrium 2SO3 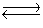 2SO2 + O2 ( Hºrxn= 198 kJ/mol) , if we increase the reaction temperature, the equilibrium will
2SO2 + O2 ( Hºrxn= 198 kJ/mol) , if we increase the reaction temperature, the equilibrium will
A) shift to the right.
B) shift to the left.
C) not shift.
D) The question cannot be answered because the equilibrium constant is not given.
Correct Answer:
Verified
Q39: The reaction 2SO3(g) Q40: For the following reaction at equilibrium, Q41: Consider this gas phase equilibrium system: Q42: A solution was prepared such that the Q43: Consider this reaction at equilibrium: 2SO2(g)+ Q45: When the substances in the equation Q46: Consider this reaction at equilibrium at a Q47: Consider the reaction N2(g)+ 3H2(g) Q48: When the reaction 2H2S(g) Q49: The equilibrium constants for the chemical![]()

![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents