A solution was prepared such that the initial concentrations of Cu2+(aq)and CN-(aq)were 0.0120 M and 0.0400 M, respectively. These ions react according to the following chemical equation
Cu2+(aq)+ 4CN-(aq) 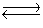 Cd(CN)42-(aq)Kc = 1.0 * 1025
Cd(CN)42-(aq)Kc = 1.0 * 1025
What will be the concentration of CN-(aq)at equilibrium?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q37: Hydrogen iodide decomposes according to the equation
Q38: Consider the following equilibria: 2SO3(g)
Q39: The reaction 2SO3(g) Q40: For the following reaction at equilibrium, Q41: Consider this gas phase equilibrium system: Q43: Consider this reaction at equilibrium: 2SO2(g)+ Q44: For the reaction at equilibrium 2SO3 Q45: When the substances in the equation Q46: Consider this reaction at equilibrium at a Q47: Consider the reaction N2(g)+ 3H2(g) ![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents