Consider the reaction N2(g)+ 3H2(g) 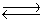 2NH3(g). The production of ammonia is an endothermic reaction. Will heating the equilibrium system increase or decrease the amount of ammonia produced?
2NH3(g). The production of ammonia is an endothermic reaction. Will heating the equilibrium system increase or decrease the amount of ammonia produced?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q73: Consider the reaction N2(g)+ 3H2(g) 
Q74: Consider the reaction N2(g)+ 3H2(g) 
Q75: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q77: Consider the chemical reaction 2NH3(g) 
Q79: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q80: Calcium carbonate decomposes at high temperatures to
Q81: For the reaction H2(g)+ I2(g) 
Q82: The equilibrium between carbon dioxide gas and
Q83: The equilibrium constant for the chemical equation
2NO(g)+
Q85: What conditions are used in the Haber
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents