Short Answer
Consider the following equilibrium,
4NH3(g)+ 3O2(g) 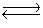 2N2(g)+ 6H2O(g)+ 1531 kJ
2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the products would increase, decrease, or remain constant after nitrogen gas was removed from the system.
Correct Answer:
Verified
Related Questions
Q86: Kc for the reaction CO2(g)+ H2(g)
Q87: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q88: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q89: Two moles of PCl5 are placed in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents