Two moles of PCl5 are placed in a 5.0 L container. Dissociation takes place according to the equation PCl5 (g) 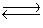 PCl3(g)+ Cl2(g). At equilibrium, 0.40 mol of Cl2 are present. Calculate the equilibrium constant (Kc)for this reaction under the conditions of this experiment.
PCl3(g)+ Cl2(g). At equilibrium, 0.40 mol of Cl2 are present. Calculate the equilibrium constant (Kc)for this reaction under the conditions of this experiment.
Correct Answer:
Verified
Q84: The equilibrium constant expression for the reaction
CuO(s)+
Q85: Equilibrium constants are known for the
Q86: Kc for the reaction CO2(g)+ H2(g)
Q87: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q88: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents