Calculate G° for the reaction 3NO2(g) + H2O(l) 2HNO3(l) + NO(g) . 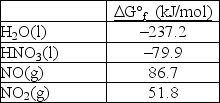
A) 8.7 kJ/mol
B) 192 kJ/mol
C) -414 kJ/mol
D) -192 kJ/mol
E) -155 kJ/mol
Correct Answer:
Verified
Q26: Aluminum forms a layer of aluminum
Q33: The equilibrium constant at 427°C for
Q34: Calculate the equilibrium constant for the
Q36: Hydrogen peroxide (H2O2)decomposes according to the
Q39: A spontaneous endothermic reaction always
A)causes the surroundings
Q39: The equilibrium constant for the reaction
Q40: For the reaction H2(g)+ S(s)
Q40: The normal freezing point of ammonia
Q41: For the reaction CuS(s)+ H2(g)
Q57: For the reaction 2C(graphite)+ H2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents