Using the thermodynamic data provided below, calculate the standard change in entropy when one mole of sodium nitrate is dissolved in water? 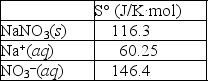 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q47: In the gas phase, formic acid
Q48: Which species will have the lowest absolute
Q48: Using the thermodynamic data provided below, calculate
Q50: For the reaction CuS(s)+ H2(g)
Q51: For the reaction CuS(s)+ H2(g)
Q53: Find the temperature at which Kp
Q54: The standard free energy of formation
Q55: Find the temperature at which Kp
Q56: The solubility product constant at 25°C
Q57: For the reaction CuS(s)+ H2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents