Using the thermodynamic data provided below, calculate the standard change in entropy when one mole of sodium sulfate is dissolved in water? 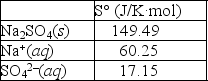 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q43: In the gas phase, methyl isocyanate
Q44: For the reaction HCONH2(g)
Q45: Find the temperature at which the
Q46: Predict the normal boiling point of triethylborane
Q47: In the gas phase, formic acid
Q48: Which species will have the lowest absolute
Q50: For the reaction CuS(s)+ H2(g)
Q51: For the reaction CuS(s)+ H2(g)
Q52: Using the thermodynamic data provided below, calculate
Q53: Find the temperature at which Kp
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents