How many protons (p) and neutrons (n) are in an atom of 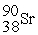
?
A) 38 p,52 n
B) 38 p,90 n
C) 52 p,38 n
D) 90 p,38 n
Correct Answer:
Verified
Q23: The number of atoms of carbon in
Q26: One mole of which element has the
Q29: What is the chemical symbol for an
Q30: How many protons (p),neutrons (n),and electrons (e)are
Q32: Which of the following two atoms are
Q34: How many protons (p)and neutrons (n)are in
Q41: What is the mass of one atom
Q49: The smallest sample of carbon atoms that
Q55: An element has two naturally occurring isotopes.One
Q58: The atoms of a particular element all
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents