What is the balanced chemical equation for the reaction of element A (unshaded spheres) with element B (shaded spheres) as represented below? 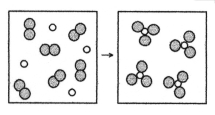
A) A + B → AB
B) 2A + 3B → 2AB
C) A + B2 → AB3
D) 2A + 3B2 → 2AB3
Correct Answer:
Verified
Q63: Q101: Assume that the unshaded spheres in the Q111: If unshaded spheres represent nitrogen atoms and Q112: What is the balanced chemical equation for Q113: What is the balanced chemical equation for Q113: Box (1)represents 1.0 mL of a solution Q115: The following diagrams represent the reaction of Q119: Reaction of A (unshaded spheres)with B2 (shaded Q120: What is the balanced chemical equation for Q121: Calcium phosphate reacts with sulfuric acid to![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents