What is the balanced chemical equation for the reaction of element A (unshaded spheres) with element B (shaded spheres) as represented below? 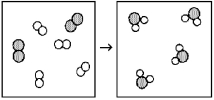
A) A + B → AB
B) 4A + 2B → 4AB
C) A2 + B2 → A2B
D) 2A2 + B2 → 2A2B
Correct Answer:
Verified
Q63: Q101: Assume that the unshaded spheres in the Q108: The following diagram represents the reaction of Q109: The following diagram represents the reaction of Q110: The following diagram represents the reaction of Q111: If unshaded spheres represent nitrogen atoms and Q112: What is the balanced chemical equation for Q113: Box (1)represents 1.0 mL of a solution Q115: The following diagrams represent the reaction of Q116: What is the balanced chemical equation for![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents