The following diagram represents the reaction of A2 (unshaded spheres) with B (shaded spheres) .What is the balanced chemical equation for this reaction,and what is the limiting reactant? 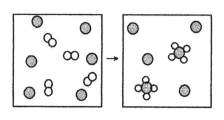
A) 2A2 + B → A4B;A2 is the limiting reactant.
B) 2A2 + B → A4B;B is the limiting reactant.
C) 4A2 + 6B → 2A4B;A2 is the limiting reactant.
D) 4A2 + 6B → 2A4B;B is the limiting reactant.
Correct Answer:
Verified
Q101: Assume that the unshaded spheres in the
Q104: If unshaded spheres represent nitrogen atoms and
Q105: Reaction of A (unshaded spheres)with B2 (shaded
Q106: The following diagrams represent the reaction of
Q108: The following diagram represents the reaction of
Q110: The following diagram represents the reaction of
Q111: If unshaded spheres represent nitrogen atoms and
Q112: What is the balanced chemical equation for
Q113: What is the balanced chemical equation for
Q116: Box (1)represents 1.0 mL of a solution
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents